
[ad_1]
Scientists have long tried to unravel the mystery of what happened to Earth’s atmosphere. Marsa planet that billions of years ago had liquid water on its surface.
Studies indicate that, about 3.5 billion years ago, that dense atmosphere, loaded with carbon dioxidebegan to disappear, leaving the red planet as the cold, dry desert we know today.
A group of MIT geologists has put forward an intriguing theory: Mars’ lost atmosphere could be trapped in the clay layers of its crust.
This idea, published in the magazine Science Advancessuggests that large amounts of carbon dioxide were sequestered on the Martian surface through geological processes that transformed CO₂ into methane, becoming trapped in clay minerals, such as smectite.
This finding not only offers a possible explanation for what happened to the atmosphere of Mars, but also opens the door to new ways to take advantage of this trapped carbon in future missions. In fact, scientists They believe that methane sequestered on the surface could be used as an energy source for future explorations and human colonizations on Mars.
The key is in the clay of Mars
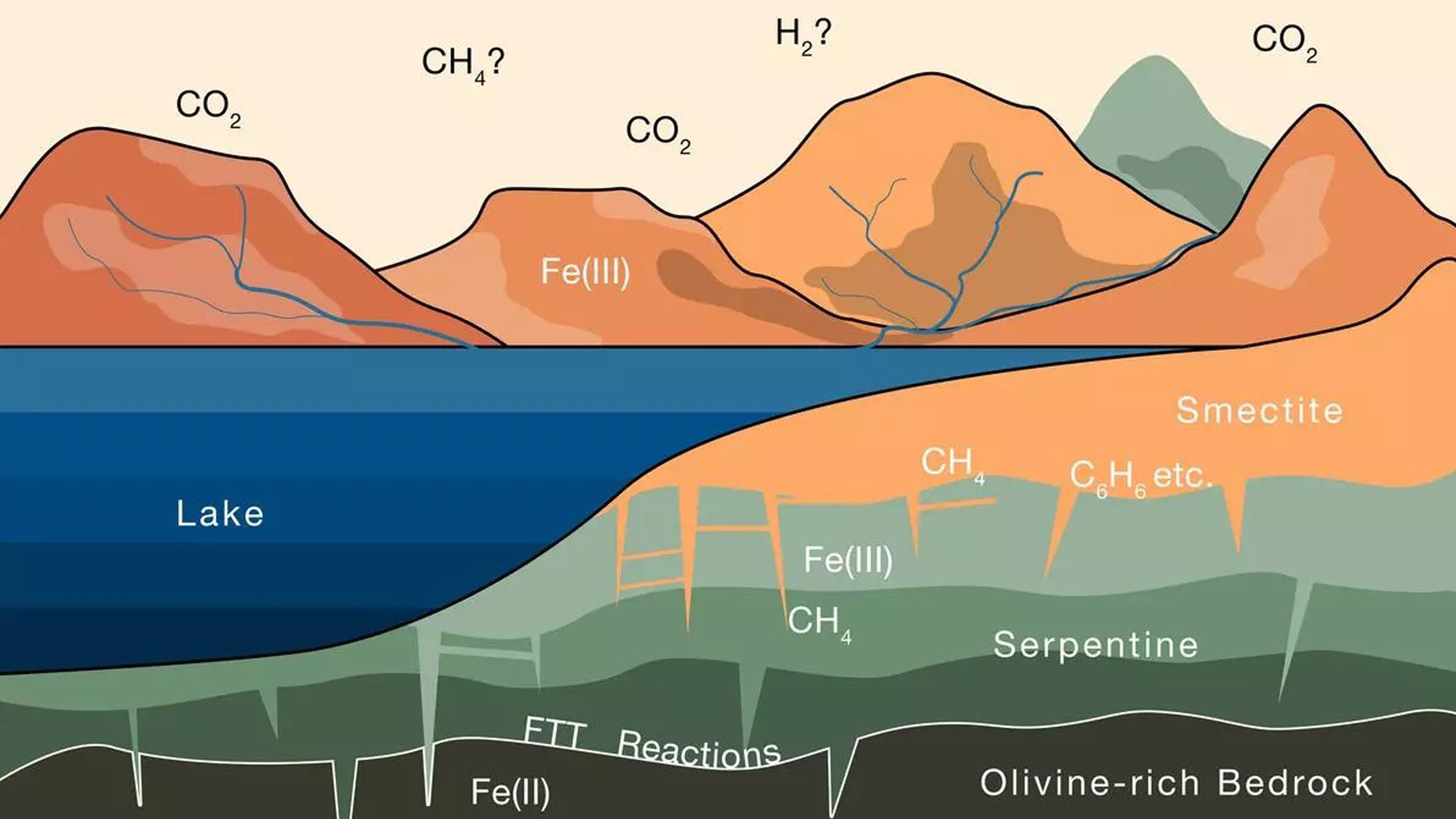
Joshua Murray, Oliver Jagoutz, et al.
The study of M.I.T. It started from a casual observation. Geologists, who were already investigating how certain clays on Earth can trap carbon dioxide, They realized that Mars is covered in similar geological formations.
On our planet, minerals such as smectite, which is formed when certain rocks interact with water and CO₂, are capable of retaining carbon for millions of years.
On Mars, the existence of large deposits of smectite and other clay minerals could have acted in a similar way. When water flowed across the Martian surface billions of years ago, these minerals could have absorbed CO₂ from the atmosphere, trapping it and transforming it into methane, which remains stored to this day.
The researchers explain that, during the early history of Mars, water on the surface interacted with rocks rich in olivine, a mineral that contains iron in a reduced form.
As water seeped through the crust, oxygen molecules in the water bonded with iron in the olivine, releasing hydrogen. This hydrogen, in turn, reacted with atmospheric carbon dioxide, forming methane.
This methane was trapped in the clay surface of Mars, specifically in minerals such as smectite, which have the capacity to store carbon for millions of years. So, The Martian atmosphere was lost, not in space, as some suggest, but rather it was sequestered on the planet’s surface..
Potential for future missions
The fact that much of Mars’ carbon dioxide is trapped in its crust not only resolves a key question about the planet’s past, but also offers opportunities for the future. According to scientists, this methane could be a source of energy in future human missions to Mars.
Like the hidden water on the Moon, Carbon trapped in Martian clays could be extracted and used as fuel. This would be a crucial step for long-duration missions, which would need local, sustainable energy sources to keep astronauts and operations running.
Although the theory presented by the MIT geologists is exciting, there is still much to discover. Future missions to Mars, such as the Perseverance rover, could look for direct evidence of these methane deposits on the surface.
Get to know how we work in ComputerToday.
Tags: POT
[ad_2]
Source link



